
Behind an unassuming doorway in an industrial park housing a Walmart and cookie-cutter office blocks lies a small clinic with a decidedly immodest goal. Its stated purpose is nothing less than to transform all of orthopedic medicine simply by tapping into the healing force within the patient’s own body.
Owner and medical director of the Centeno-Schultz Clinic, Dr Chris Centeno, a 51-year-old pain management specialist, is quietly transforming orthopedic medicine after pioneering a technique that uses the patient’s own stem cells to restore damaged tissue – cartilage, bone, ligaments and tendons – largely ending the need for surgery.
A typical patient of his is Joe Maroon, whose knee was giving way to osteoarthritis and so was being faced with an impossible choice. After years of running, biking and swimming, Maroon’s cartilage had significantly deteriorated, causing constant pain. Doctors told the 63-year-old triathlete that he needed knee replacement surgery, but as a doctor himself, a neurosurgeon at the University of Pittsburgh, he was well aware of what that might entail. Had he been just a few years younger, his doctor might not have even presented that option; artificial hips or knees wear out after about a decade and then need to be replaced again, so doctors like their patients to defer that first operation for as long as possible.
The only alternative was a lifetime of steroid injections and the overwhelming likelihood of having to end his competitive sporting activities.
During his years of waiting, Maroon chanced upon the Centeno-Schultz Clinic’s orthopedic alternative Regenexx™, which relies on using a patient’s own stem cells to heal damaged joints. Maroon was impressed enough to travel to the tiny clinic in Broomfield, a suburb outside of Denver, Colorado, to consult with the specialist who’d pioneered what he was touting as an alternative to surgery.
After some of Joe’s stem cells were extracted from his bone marrow, they were cultured and multiplied in the laboratory over several weeks before being reinjected into his damaged knee. The result was such a reduction of pain that Maroon, by that time aged 68, was able to compete in the Ironman Hawai’i triathlon six months later.
The doctor behind Joe’s remarkable recovery is pioneering a new kind of orthopedics, one that avoids surgery and relies on the patient’s own body to heal. Centeno was profoundly dissatisfied with the state of orthopedic medicine, and its reliance on steroids and surgery. Most joint issues are caused by deterioration of cartilage – which cushions the movement of bones, especially in the hips and knees – usually due to inflammation. As cartilage is poorly supplied with blood, it ordinarily doesn’t regenerate.
Medicine makes tacit recognition of this fact with the few alternatives to joint replacement it offers. Surgery to repair cartilage either attempts to ‘injure’ it to prompt the bone beneath it to initiate a repair response, although such procedures often don’t work very well,1 or chunks of healthy cartilage are implanted into areas of damage as a form of tissue engineering.2 Both have a spotty record of success, mostly because the transplants are often destroyed by the body’s own natural inflammatory response.
After the turn of the millennium, Centeno became interested in animal research on stem cells,3 and wondered whether it might apply to people too. The research was showing that when damaged joints were injected with the animal’s own stem cells, the cells – as if responding to some hidden blueprint – as well as the tissue surrounding them would differentiate into the appropriate tissues required to heal the damage. Even more encouraging, the tissue continued to do its repair job over time.
Centeno wanted to test whether the ready supply in the bone marrow of most patients of malleable mesenchymal stem cells (MSCs), which are already likely to turn into bone, cartilage and connective tissue cells, could be used to rebuild damaged joints. Centeno’s ‘ah-ha’ moment came when he realized that adding to the brew a solution of the patient’s own blood platelets would ‘supercharge’ the MSCs to both replicate and also to differentiate into more cartilage and bone to repair the joint.
Centeno partnered with Dr John Schultz, an orthopedic specialist and anesthesiologist, and the Centeno-Schultz Clinic opened its doors in Broomfield. Early on, Centeno had also decided to substantiate the clinic’s work by carrying out painstaking research and follow-up on all their patients and publishing the findings, ultimately spending $500,000 of his own funds on mainly research programs. So far, Centeno has carried out more research on stem-cell orthopedic repair than has any other research center.
In 2008, he published the results of the first patient to undergo his procedure. His guinea pig was a man who’d suffered for years from knee pain that had not improved with surgery.
Centeno harvested MSCs from the patient’s hip bone (see box, page 64), and multiplied and ‘boosted’ them by culturing the cells with tissue factors from the patient’s blood platelets. After a few days, he injected this brew into the patient’s knee.
The results were unequivocal. Just a month after the procedure, the patient’s knee-cartilage surface area had expanded by more than 20 percent, and the joint meniscus – the cushiony cartilaginous pad that bears the brunt of the thigh bone’s weight – was also 29 percent larger after six months.4 The patient’s previously limited range of motion was now nearly normal and his pain, formerly assessed as 4 out of a possible 10, had plummeted to 0.4.
Impressive results
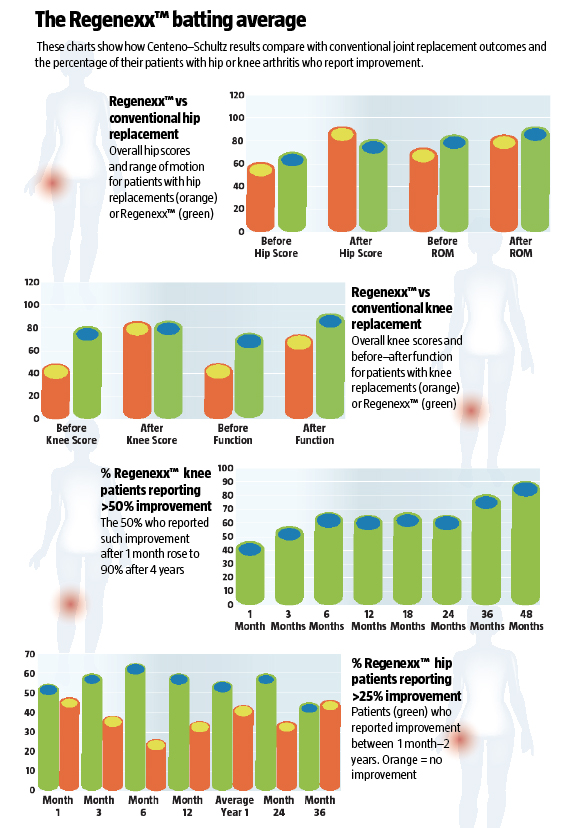
In the intervening six years, Centeno and his colleagues (including doctors he has trained around the world) have performed some 10,000 procedures on all manner of orthopedic and soft-tissue injuries, hundreds of them involving patients with diseased knee and hip joints. The results are impressive – even more so because his patients continue to improve over time, month after month, year after year. Recently, his registry data showed that, of 221 overweight (body mass index scores of more than 25 kg/m2) and older patients with knee arthritis, 80 percent recorded more than 25 percent improvement after the operation, with an average of nearly 60 percent improvement after two years. And of 999 middle-aged people who were only slightly overweight, the figures showed that 90 percent reported a more than 50 percent improvement , with more than 70 percent average improvement after four years. Although the results for hip pain are not as spectacular, more than 60 percent of such patients still reported more than 25 percent pain relief, with an average improvement of 42 percent in patients under 55 (22 percent if older than 55).
Puzzling over why hip patients don’t do as well as knee patients, the Regenexx™ team studied their post-treatment registry and discovered that a patient’s total range of hip motion was connected to outcome success; the poorer the range of motion, the poorer the outcome of the standard treatment (where injections are the patient’s own stem cells are just injected back in).
Nevertheless, both hip and knee stem-cell patients fare well when compared with joint-replacement patients. According to an independent comparison made by American orthopedic surgeon Dr Mitch Sheinkof, patients getting hip replacements showed a greater improvement in the Harris hip test (which measures pain and movement ability), but Regenexx™ patients enjoyed better range of motion and a better overall risk/benefit equation, as the stem cell procedure is far less invasive and carries far less risk. Some 73 percent of the hip Regenexx™ patients were able to return to sports activities. Knee patients in particular show greater overall functional improvement.
In 2011, Centeno and his team publ
ished a safety and complications report of 339 patients, most of whom had arthritis of the knee and all of whom had been told they needed knee replacements. After receiving the stem-cell Regenexx™ treatment, only 4.1 percent of these patients went on to get an artificial knee, while the rest did well enough with the Regenexx™ treatment to avoid surgery.5
Besides diseased joints, Centeno’s clinic handles all kinds of soft tissue and spinal injuries. Spending an afternoon listening to stories in the busy clinic’s waiting room is instructive. In a typical case, an elderly woman so damaged with arthritis she could barely walk says she’s become a mountain climber since having the procedure; another woman, whose knee anterior cruciate ligament was damaged 20 years ago playing basketball, has seen it repaired with the stem-cell procedure. Patients with rotator-cuff injuries, tendonitis, spinal injuries, deteriorating discs – all are open to repair without the often catastrophic effects of surgery.
“With this type of medicine, we can move from managing pain to actually curing the injured tissue,” says Centeno. “Imagine – knees, hips, backs all fixed. No more pain. No more surgery. No more meds. All you need are your own stem cells. Doctors would start actually curing disease instead of prescribing drugs to manage disease, and that’s a groundbreaking paradigm shift.”
Not surprisingly, the US Food and Drug Administration (FDA) doesn’t like this paradigm shift one little bit. Autologous (donor and recipient are the same person) stem-cell therapy not only threatens to revolutionize orthopedic medicine as we know it, but it also threatens to wipe away some of the $40 billion pain-management drug business and to ultimately rock the foundations of FDA control of the entire drug marketplace.
The FDA, now largely funded by the pharmaceutical industry, has visited the Colorado clinic multiple times, sifting through Centeno’s laboratory “as if it were a mass drug manufacturing factory,” he says – and unlike most doctors, he decided not to take this harassment lying down. In August 2010, the Center for Biologics Evaluation and Research, a division of the FDA, filed an injunction against Centeno, ordering his clinic to stop culturing patients’ stem cells. In response, Centeno filed for multiple temporary restraining orders simultaneously in Denver and in Washington, DC. The FDA then issued an injunction against the clinic, whereupon Centeno abandoned the restraining orders and promptly countersued the FDA for interfering with his trade.
The FDA maintains that extracting, manipulating and culturing a person’s stem cells constitutes a ‘cultured drug product’ not unlike culturing an antibiotic. In 2012, in an attempt to control this new medical technique, the FDA ruled that your own stem cells should now be considered a ‘drug’ subject to the agency’s control.
The FDA’s ruling was held up in a court case, even though Centeno argued that “stem cells are body parts and not the property of the government or Big Pharma.”
“What we’re doing in our medical practice is no different, in principle, than a fertility clinic that uses in-vitro fertilization,” says Schultz.
At the time, this meant that Centeno’s clinic could only treat those parts of the body that receive enough blood flow to benefit from an ordinary stem-cell injection – harvested from the hip and immediately injected into the damaged joint.
As Centeno and Schultz were no longer able to culture stem cells within the US, they were forced to move that part of their practice to the Cayman Islands, which is outside of FDA interference. In most cases, this is not an impediment to the work in the Colorado clinic, as 90 percent of their practice concerns situations where patients can benefit from having their existing MSCs harvested and injected back in. Any patients with problems requiring a larger batch of stem cells now will travel to the clinic in the Caymans, funds permitting.
Rulings and riders
One noted author and philosopher recently damaged her knee ligaments, and the Denver doctors advised her to make the trip to the Caymans to repair it. But Sharon Martin wasn’t as fortunate. In 2009, the then 48-year-old Wyoming resident was treated at the Colorado clinic for degenerated discs in her lower back. The procedure eliminated half her pain, but by the time she’d phoned to schedule a repeat procedure, the FDA had imposed its ruling. Although her stem cells had already been harvested, they’ve remained frozen and in storage. A trip to the Caymans is now her only option for full relief of her pain, which may be beyond her budget.
Possibly because research has shown that the native stem cells in hips aren’t as robust as those in knees and have less of an in-built repair mechanism, older hip patients tend to do far better with cultured stem cells than ones just taken out of the patient’s bone marrow and reinjected back in.
Last October, the FDA published a rider to clarify its initial ruling, allowing work like Centeno’s to carry on so long as the harvested stem cells are returned to the patient without too much manipulation and as part of the ‘same’ procedure. This amounted to a cautious stamp of approval for the the clinic to carry on its work with damaged joints, using a procedure that simply harvests MSCs from the hip bone, then purifies them and injects them directly into the damaged joint.
A number of other orthopedic specialists are also using stem-cell technology, albeit without the weight of published evidence that Centeno brings to his work. Many of his colleagues who attend his lectures or training sessions have called him a visionary, even a genius. As with most non-drug breakthroughs in medicine, Centeno’s primary obstacle is not the challenges presented by his patients, but the immense power of the Pharma-led corporate Medical Establishment.
Despite the FDA’s challenges, Centeno remains confident that the sheer weight of evidence of the safety and success of his procedure will speak for itself – and prevail. For the sake of the millions of patients not helped by conventional approaches, let’s hope he’s right.
M is for mesenchymal
Stem cells are nothing less than shape-shifters – precursor cells that go on to differentiate into whatever kind of cell tissue is required. Stem cells have most controversially been harvested from human embryos and adipose (fat) tissue, but those with the best record of success and safety for treating joint problems are the so-called mesenchymal stem cells (MSCs), found in large numbers in bone marrow tissue. Although other clinics use stem cells from adipose tissue in joints, Centeno believes that MSCs are superior because these cells are already partially committed to becoming bone, muscle, ligament or tendon, and are easily harvested from bone marrow and reproduce rapidly, making them ideal candidates for repairing those very structures.1
According to Centeno, under certain conditions, MSCs can be prompted to differentiate into the specific sort of tissue needed; when implanted into affected joints, these cells work to repair cartilage and bone, and even the connective tissues in between. Indeed, there’s even evidence they can protect against inflammation-related tissue damage and have the ability to modulate autoimmune responses too.2
Can stem cells cause cancer?
It’s well known that injecting embryo-derived stem cells into a patient can cause tumors to develop. For this reason, Centeno and his team as well as others have kept careful follow-up data on patients receiving MSCs for orthopedic purposes. Recently, Centeno published follow-up data on 227 patients who’d been tracked for up to four years, many of whom were later scanned for procedure-related problems, including tumors
– the largest study of its kind and the first of other similar studies (see main story).1 No cancerous growths or formations were found.
In a separate study, Japanese researchers went even further, following 45 patients, who had received MSC transplants to repair cartilage, for more than 11 years. They also could find no evidence of either tumors or infections.2
What happens at the clinic
The Centeno-Schultz Clinic occupies a small suite of offices in Broomfield, Colorado, a suburban sprawl of shopping malls and office blocks halfway between Denver and mountainous Boulder, where Staples has its head offices, as do a few other chain-store giants. The center of town and Broomfield life is the Flatiron Crossing Mall, sitting against a backdrop of spectacular snow-capped mountains. Nevertheless, the Centeno-Schultz Clinic treats patients from all over the world who stay in nearby hotels to undergo what is typically an eight-day procedure.
Before making the patient’s appointment, one or more of the clinic’s doctors examines the previous magnetic resonance imaging (MRI) scans to determine the patient’s eligibility for stem-cell procedures.
If the patient is determined to be someone the clinic can help and the patient chooses to go ahead with the procedure, he should plan for an eight-day stay.
On the first day, one or more of the doctors in the X-ray team examines the patient’s joints with ultrasound and possibly arthroscopy. In the case of knees, the doctor inserts a six-inch needle with an attached camera into the joint to see what’s going on. The doctors also administer prolotherapy, which involves injecting an inflammatory agent to stimulate a natural healing response.
Several days later, the patient undergoes a blood draw and the stem-cell harvesting procedure, which entails the doctor essentially hammering into an iliac crest – one of the curved ‘wings’ on either side of the pelvis or hip bone – to extract cells from the bone marrow. Several hours later, the stem cells are ready to be reinjected into the damaged joint under the guidance of ultrasound, during which the patient usually elects conscious sedation.
On the seventh day, the patient returns for more blood-platelet extraction and injections to ‘supercharge’ the stem-cell implant. For many patients, a single visit is sufficient, while others may have to come back for multiple procedures.
The cost of the full eight-day treatment is around $7,500, which is generally not covered by medical insurance, plus any hotel and travel costs for out-of-towners. For more information, visit www.centenoschultz.com, or www.regenexx.com for a list of doctors trained in the procedure.
The Regenexx™ batting average
These charts show how Centeno–Schultz results compare with conventional joint replacement outcomes and the percentage of their patients with hip or knee arthritis who report improvement.
Main article
|
References |
|
|
1 |
Eur Cell Mater, 2005; 9: 23–32 |
|
2 |
J Bone Joint Surg Am, 2010; 92: 994–1009; Arthritis Res Ther, 2009; 11: 211 |
|
3 |
J Bone Joint Surg Br, 2001; 83: 289–94 |
|
4 |
Pain Physician, 2008; 11: 343–53 |
|
5 |
Curr Stem Cell Res Ther, 2011; 6: 368–78 |
Can stem cells cause cancer?
|
References |
|
|
1 |
Curr Stem Cell Res Ther, 2010; 5: 81–93 |
|
2 |
J Tissue Eng Regen Med, 2011; 5: 146–50 |
M is for mesenchymal
|
References |
|
|
1 |
Curr Opin Biotechnol, 2004; 15: 406–10; Pain Physician, 2008; 11: 343–53 |
|
2 |
Arthritis Res Ther, 2008; 10: 223; 2009; 11: 211 |
What do you think? Start a conversation over on the... WDDTY Community